Progesterone (P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the major progestogen in the body. And has a variety of important functions in the body. It is also a crucial metabolic intermediate in the production of other endogenous steroids, including the sex hormones and the corticosteroids, and plays an important role in brain function as a neurosteroid.
Medical use
Main articles: Progesterone (medication), Pharmacodynamics of It, and Pharmacokinetics of It.
progesterone tablets is used as a medication. It is used in combination with estrogens mainly in hormone therapy for menopausal symptoms and low sex hormone levels in women. It may also be used alone to treat menopausal symptoms. Studies have shown that transdermal progesterone (skin patch) and oral micronized It are effective treatments for certain symptoms of menopause such as hot flashes and night sweats, which are otherwise referred to as vasomotor symptoms or VMS.
It is also used in women to support pregnancy and fertility and to treat gynecological disorders.It has been shown to prevent miscarriage in women with 1) vaginal bleeding early in their current pregnancy and 2) a previous history of miscarriage. It can be taken by mouth, through the vagina, and by injection into muscle or fat, among other routes.
progesterone function
2. It has the power to promote the thickening of the lining of the uterus, promote the bending of the gland and to increase secretion function.
3. It can inhibit the peristalsis of the uterus, and contribute to the cervix contraction, secretion of mucus, etc.. These physiological changes provide suitable environment for the operation. Growth and development of early embryos, as well as the continued growth of the fetus.
5. It is involved in the feedback regulation of the hypothalamus and anterior pituitary. Which makes the balance of the animal reproductive hormones. In vivo, progesterone content of all sorts of livestock follicular phase is below 1 ng/ml. While in bovine corpus luteum period is approximately 4 ng/ml, pregnancy period is about 18 ng/ml.
6. Formerly biochemical study shows that It modulates action as progestogens, clinical for the treatment of habitual abortion, dysmenorrhea, amenorrhea and other symptoms. One of progesterone’s most important functions is as hormone drugs, to promote and maintain the uterine changes in the early stage of pregnancy, used in habitual abortion, irregular menstruation, etc.. In addition, It also behaves as steroid hormone drug as well as progestogens, which is used in treatment of threatened abortion.
Description
Chemical Properties White powder. Melting point 121°C. Stable in air. Insoluble in water. A female sex hormone. Low toxicity.
Occurrence Colchicum luteum also yields this alkaloid.
Uses Prog is used as a contraceptive, for amenorrhea, for premenopausal syndrome, infertility, incomplete pregnancies, and anovulatory uterine bleeding.
Uses Steroid hormone produced by the corpus luteum. Induces maturation and secretory activity of the uterine endothelium; suppresses ovulation. It is implicated in the etiology of breast cancer.This compound is a contaminant of emerging concern (CECs).
Definition
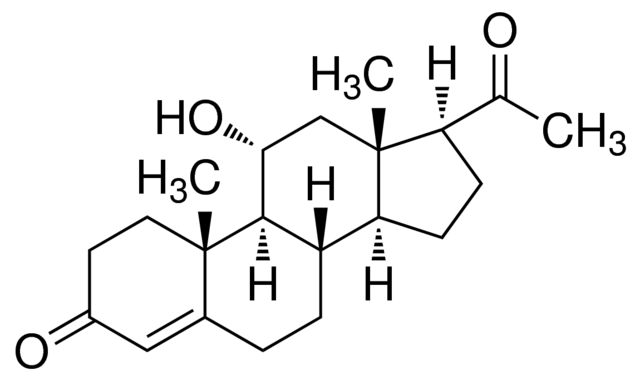
IUPAC name
Pregn-4-ene-3,20-dione
Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,11aS)-1-Acetyl-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one
Other names
P4; Pregnenedione
More Introduction:https://en.wikipedia.org/wiki/Progesterone