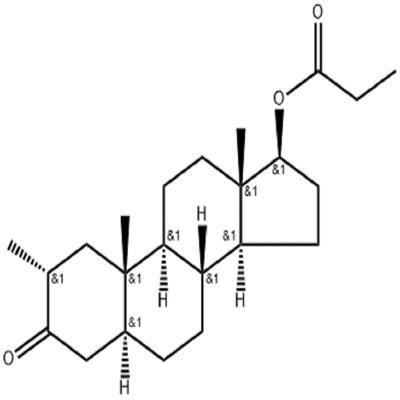
Drostanolone propionate (masteron) is an injectable anabolic steroid (DHT) derived from dihydrotestosterone. Here, the main chain of DHT is modified with a 2-methyl group to increase its anabolic properties, making the compound more effective than the unmethylated parent in promoting muscle tissue growth. Drostanolone Propionate is described in the product literature as a “steroid with powerful anabolic and anti-estrogenic properties”, and its anabolic properties are relatively mild, especially when used in combination with other anabolic drugs. This drug is most commonly used by bodybuilders who are losing fat and athletes with high agility requirements, as it greatly promotes the gain of lean muscle mass, and also powerful strength gains, while this process is usually accompanied by a reduction in body fat levels and very few side effects.
Masteron Effects
It is no surprise that Masteron is best used during a fat loss cycle. However, it requires that the user’s body fat is very low. This is why it is usually used at the end of the preparation period, when the athlete’s body fat is already very low. Masteron can help remove the last bit of stubborn fat. If your body fat is not as low as an athlete’s standard, the effect will not be as obvious. The best effect is to have a body fat below 10%.
As a “powerful” male hormone, Masteron is very suitable for athletes who are looking to increase strength. It can greatly improve muscle strength, endurance and recovery speed without adding muscle.
Masteron has no effect during a muscle-building steroid cycle. Although it is possible to increase circumference with high-dose injections, there are too many other steroids that are more effective than it. Some people inject drostanolone during the muscle-building period to avoid fat gain, but this is not a good reason. Sebum should be controlled by diet and training. At the same time, although Masteron has an anti-estrogen effect, it is not strong enough to counteract the estrogenic effect caused by large amounts of exogenous testosterone in the off-season muscle-building cycle and the estrogenic reaction caused by drugs such as Dianabol.
Drostanolone Propionate Side Effects
Drostanolone propionate side effects include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire. It has no risk of liver damage. The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has moderate anabolic effects and weak androgenic effects, which give it a mild side effect profile and make it especially suitable for use in women. The drug has no estrogenic effects. Drostanolone propionate is an androgen ester and a long-lasting prodrug of drostanolone in the body.
Drostanolone propionate uses in bodybuilding and drostanolone propionate dosage (for men)
To enhance fitness or performance, the drug is usually injected 2-3 times per week. The total weekly dose is usually 400-800 mg for 6-12 weeks. This dosage and cycle is enough to make a very large improvement in lean muscle mass and strength.
Drostanolone Propionate is often combined with other steroids to enhance the effects. Common Cycle combinations include injectable anabolic steroids such as Deca-Durabolin or Equipoise can bring about significant muscle growth without excessive water retention. To increase muscle mass, they are often combined with injectable testosterone. Compared to using these steroids alone (usually at higher doses), the combination results in firmer muscles with lower water retention and other estrogenic side effects. However, Drostanolone Propionate is most commonly used in the fat loss and preparation phase. Here, it is usually used in combination with other non-aromatizable steroids (such as Winstrol®, Primobolan®, Parabolan, or Anavar), which can greatly aid in muscle retention and fat loss.
Drostanolone Propionate Usage (Females)
When used to enhance fitness or performance, the most common dosage is 50mg per week for 4 to 6 weeks. Doses equal to or less than 100mg per week are rarely associated with side effect symptoms. Please note that due to the short-acting nature of propionate, the total weekly dose can usually be divided into multiple injections every two or three days.
Drostanolone propionate Chemical Properties
Melting point 114-120°C
alpha D +24°
Boiling point 432.53°C (rough estimate)
density 1.0669 (rough estimate)
refractive index 1.4700 (estimate)
storage temp. Controlled Substance, -20°C Freezer
solubility DMF:30.0(Max Conc. mg/mL);83.21(Max Conc. mM)
DMSO:24.0(Max Conc. mg/mL);66.57(Max Conc. mM)
Ethanol:2.0(Max Conc. mg/mL);5.55(Max Conc. mM)
form A crystalline solid
InChI InChI=1/C23H36O3/c1-5-21(25)26-20-9-8-17-16-7-6-15-12-19(24)14(2)13-23(15,4)18(16)10-11-22(17,20)3/h14-18,20H,5-13H2,1-4H3/t14-,15+,16+,17+,18+,20+,22+,23+/s3
InChIKey NOTIQUSPUUHHEH-DEMPVMEFNA-N
SMILES C[C@]12C[C@@H](C)C(=O)C[C@]1([H])CC[C@@]1([H])[C@]3([H])CC[C@H](OC(=O)CC)[C@@]3(C)CC[C@]21[H] |&1:1,3,8,12,14,18,24,28,r|
CAS DataBase Reference 521-12-0(CAS DataBase Reference)
NIST Chemistry Reference Dromostanolone propionate(521-12-0)
More Introduction:https://en.wikipedia.org/wiki/Drostanolone_propionate