Obesity is an important risk factor for heart failure (HF), especially for heart failure with preserved ejection fraction (HFpEF). Obesity can lead to a systemic inflammatory state, which may affect the myocardium through inflammatory transformation of epicardial adipose tissue. As body mass index (BMI) increases, the risk of heart failure also increases.
Weight loss interventions (such as gastric bypass surgery and GLP-1 receptor agonist therapy) can reduce systemic inflammation, reduce epicardial fat volume, reduce the risk of new heart failure, and relieve symptoms in established HFpEF patients.
Tirzepatide is a long-acting dual agonist of glucose-dependent insulin secretagogue polypeptide (GIP) and GLP-1 receptor that can significantly reduce the weight of obese patients, but there is a lack of data on its effect on cardiovascular outcomes.
Main research content and results
The SUMMIT study is an international, double-blind, randomized, placebo-controlled trial. The researchers randomly assigned 731 patients with heart failure (ejection fraction at least 50% and BMI at least 30) in a 1:1 ratio to Tirzepatide (subcutaneous injection once a week, up to 15 mg) or placebo for at least 52 weeks. The primary endpoint was the composite endpoint of cardiovascular death or worsening heart failure (first event analysis) and the change in the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) from baseline to 52 weeks.
Tirzepatide treatment effect:
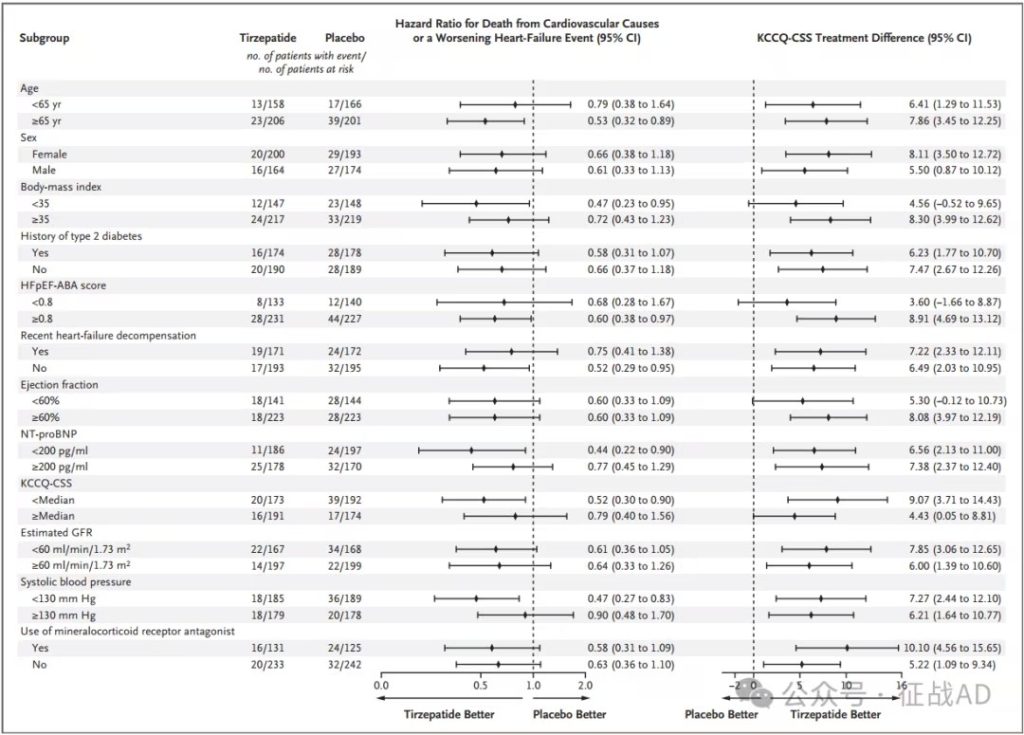
Reduced risk of cardiovascular events and death: After a median follow-up of 104 weeks, the Tirzepatide treatment group showed a lower composite risk of cardiovascular death or worsening heart failure events (9.9% vs 15.3%) compared with the placebo group, with a hazard ratio of 0.62 (95% CI, 0.41 to 0.95; P = 0.026).
Reduced heart failure worsening events: The incidence of heart failure worsening events in the Tirzepatide group was lower than that in the placebo group (8.0% vs 14.2%), with a risk ratio of 0.54 (95% CI, 0.34 to 0.85).
Improved quality of life: At 52 weeks, the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score (KCCQ-CSS) in the Tirzepatide group increased by an average of 19.5 points, while the placebo group increased by 12.7 points, with a difference of 6.9 points between the groups (95% CI, 3.3 to 10.6; P<0.001), indicating that Tirzepatide treatment significantly improved the quality of life of patients.
Significant weight loss: At 52 weeks, the average weight loss in the Tirzepatide group was 13.9%, while the placebo group only decreased by 2.2%, with a difference of 11.6 percentage points between the groups (95% CI, -12.9 to -10.4; P<0.001).
Improved exercise tolerance: The average 6-minute walk distance in the Tirzepatide group increased by 26.0 meters, while the placebo group increased by 10.1 meters, with a median difference of 18.3 meters between the groups (95% CI, 9.9 to 26.7; P<0.001).
Reduced inflammatory markers: The average high-sensitivity C-reactive protein (hsCRP) level in the Tirzepatide group decreased by 38.8%, while the placebo group only decreased by 5.9%, with a difference of 34.9 percentage points between the groups (95% CI, -45.6 to -22.2; P<0.001).
Safety: 6.3% of patients in the Tirzepatide group discontinued the trial drug due to adverse events (mainly gastrointestinal reactions), while 1.4% in the placebo group.
The results of the study showed that Tirzepatide can not only significantly improve cardiovascular prognosis in patients with HFpEF and obesity, but also improve their quality of life and exercise tolerance, while reducing body weight and inflammatory marker levels.