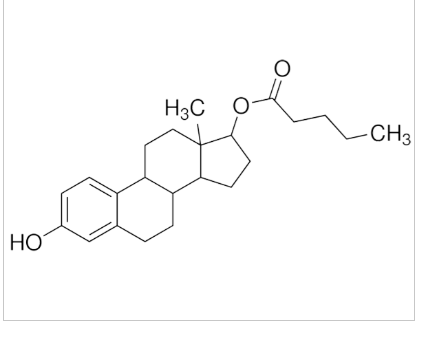
Estradiol valerate (EV), sold for use by mouth under the brand name Progynova and for use by injection under the brand names Delestrogen and Progynon Depot among others, is an estrogen medication. In women, it is used in hormone therapy for menopausal symptoms and low estrogen levels, hormone therapy for transgender women, and in hormonal birth control. It is also used in the treatment of prostate cancer in men. The medication is taken by mouth or by injection into muscle or fat once every 1 to 4 weeks.
Chemical Properties White to off-white crystalline powder, odorless. Insoluble in water, easily soluble in ethanol, acetone, chloroform, slightly soluble in vegetable oil.
Uses It is a female hormone (estrogen).estradiol valerate injection It is used to reduce symptoms of menopause which are vaginal dryness, hot flashes, and others. The symptoms of menopause manifest because the body is making less estrogen; with It, these symptoms are brought to the lowest minimum. This medication therapy can also produce enough estrogen in women with ovarian failure, hypogonadism and can be used by men to treat prostate cancer (palliation only).
Therapeutic Function Estrogen
General Description It is a synthetic hormone that is extensively metabolized to estradiol and valeric acid before reaching the systemic circulation. It is well suited for treatment of the characteristic symptoms accompanying menopause in women.
estradiol valerate side effects As with all pharmaceutical medicines, some unwanted effects can occur from the use of It.
Always consult a physician for medical advice before use.
Common side effects may include: bloating, vomiting, nausea, breast tenderness, headache, weight changes, and mood changes.
More severe side effects may include mental changes, breast lumps, vaginal bleeding, liver damage, increased thirst, and sudden vision loss.
Safety Profile Suspected carcinogen with carcinogenic and teratogenic data. Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating fumes.
Other names EV; E2V; Oestradiol valerate; Estradiol pentanoate; Estradiol valerianate
Routes of administration By mouth, sublingual, intramuscular injection,[2] subcutaneous injection
Drug class Estrogen; Estrogen ester
ATC code G03CA03 (WHO)
Bioavailability Oral: 3–5%
IM injection: 100%
Protein binding Estradiol: ~98% (to albumin and SHBGTooltip sex hormone-binding globulin)
Metabolism Cleavage via esterases in the liver, blood, and tissues
Metabolites Estradiol, valeric acid, and metabolites of estradiol
estradiol valerate half life
Elimination half-life Oral: 12–20 hours (as E2)
IM injection: 3.5 (1.2–7.2) days
Duration of action IM injection:
5 mg: 7–8 days
10 mg: 10–14 days
40 mg: 2–3 weeks
100 mg: 3–4 weeks
Excretion Urine (80%)
Melting point 144 to 145 °C (291 to 293 °F)
More Introduction:https://en.wikipedia.org/wiki/Estradiol_valerate